Abstract
Background
Enhancer of zeste homolog 2 (EZH2) and its close homolog, EZH1, catalyze the attachment of 3 methyl groups to histone H3 at lysine 27 (H3K27me3). H3K27me3 is an epigenetic marker involved in downregulating gene expression associated with tumor suppression and cell differentiation. Both altered EZH2 expression and EZH1's compensatory activity have been implicated in the development and progression of non-Hodgkin lymphomas (NHLs), including PTCL and ATL. R/R PTCL and ATL are associated with inferior outcomes, and many patients (pts) are not eligible for potentially curative transplants.
Valemetostat tosylate (DS-3201b; also known as valemetostat) is a novel, potent, and selective dual inhibitor of EZH2 and EZH1. A first-in-human phase 1 study was conducted for pts with R/R NHL in Japan and the US. Valemetostat demonstrated clinical antitumor activity in pts with NHL, including R/R PTCL and R/R ATL. Treatment with valemetostat 150 or 200 mg/day led to overall response rates (ORRs) of 54.5% (95% CI, 38.8%-69.9%) and 57.1% (95% CI, 28.9%-82.3%) in pts with R/R PTCL (n=44) or R/R ATL (n=14), respectively (EHA 2021. Abstract S218). Durability of response was demonstrated by a median duration of response (DOR) of 56.0 weeks (range, 44.43- -) in PTCL pts. Based on these encouraging efficacy results, a global phase 2 study was designed.
Methods
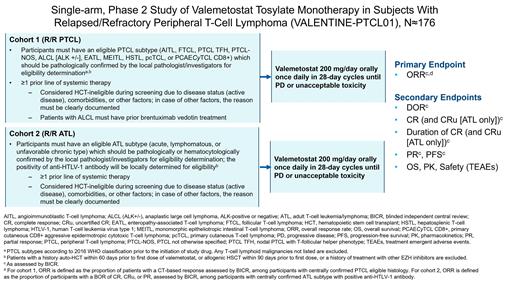
VALENTINE-PTCL01 (NCT04703192) is a global, multicenter, open-label, single-arm, noncomparative, 2-cohort, phase 2 study designed to evaluate the efficacy and safety of valemetostat monotherapy in adult pts with R/R PTCL or R/R ATL. Pts with R/R PTCL or R/R ATL are independently enrolled in cohort 1 or 2, respectively (Figure). Eligibility for both cohorts is determined based on a diagnosis made by a local pathologist/investigator and is centrally confirmed.
Pts eligible for cohort 1 must have 1 of the following R/R PTCL subtypes: (1) angioimmunoblastic T-cell lymphoma, (2) follicular T-cell lymphoma, (3) nodal PTCL with T-follicular helper (TFH) phenotype, (4) PTCL not otherwise specified, (5) ALK-positive anaplastic large cell lymphoma (ALCL), (6) ALK-negative ALCL, (7) enteropathy-associated T-cell lymphoma, (8) monomorphic epitheliotropic intestinal T-cell lymphoma, (9) hepatosplenic T-cell lymphoma, (10) primary cutaneous γ-δ T-cell lymphoma, or (11) primary, cutaneous, CD8+, aggressive, epidermotropic, cytotoxic T-cell lymphoma. Pts must have ≥1 measurable lesion as assessed by computed tomography (CT). Pts eligible for cohort 2 must have acute, lymphomatous, or unfavorable, chronic-type R/R ATL with evaluable abnormal lymphocytes in the peripheral blood and skin lesions. Pts in both cohorts must have received ≥1 prior line of systemic therapy and have adequate organ function prior to the first dose of valemetostat. Pts with ALCL must have received prior brentuximab vedotin treatment. Pts who progressed after autologous or allogeneic hematopoietic cell transplant are eligible. Biomarker positivity (eg, EZH2 mutation) is not required for inclusion. Pts with active central nervous system involvement are excluded.
Valemetostat 200 mg/day is administered orally once daily until disease progression or unacceptable toxicity occurs. The primary endpoint is ORR with valemetostat monotherapy as assessed by blinded independent central review. Pts in cohort 1 will be assessed by CT response criteria according to the 2014 Lugano criteria (J Clin Oncol. 2014;32:3059-68). Pts in cohort 2 will be assessed by the modified 2009 ATL criteria stemming from an international consensus meeting (J Clin Oncol. 2009;27:453-59). Secondary endpoints include DOR, complete response (CR) rate, duration of CR, partial response rate, progression-free survival, overall survival, pharmacokinetics, and safety and tolerability of valemetostat. Safety endpoints include treatment-emergent adverse events (TEAEs); TEAEs of special interest; serious TEAEs; fatal events; TEAEs leading to treatment discontinuation, interruption, or reduction; laboratory assessments; vital signs; and electrocardiogram analyses. VALENTINE-PTCL01 is currently recruiting at multiple sites in North America, Europe, Asia, and Oceania.
Figure. VALENTINE-PTCL01 Study Design
Foss: Daiichi Sankyo: Honoraria; Kura: Honoraria; Acrotech: Honoraria, Speakers Bureau; Seattle Genetics: Honoraria, Speakers Bureau; Kyowa: Honoraria; Mallinckrodt: Honoraria. Porcu: Viracta: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Innate Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; Daiichi: Honoraria, Research Funding; Kiowa: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Spectrum: Consultancy; DrenBio: Consultancy. Horwitz: ADC Therapeutics, Affimed, Aileron, Celgene, Daiichi Sankyo, Forty Seven, Inc., Kyowa Hakko Kirin, Millennium /Takeda, Seattle Genetics, Trillium Therapeutics, and Verastem/SecuraBio.: Consultancy, Research Funding; Affimed: Research Funding; Aileron: Research Funding; Acrotech Biopharma, Affimed, ADC Therapeutics, Astex, Merck, Portola Pharma, C4 Therapeutics, Celgene, Janssen, Kura Oncology, Kyowa Hakko Kirin, Myeloid Therapeutics, ONO Pharmaceuticals, Seattle Genetics, Shoreline Biosciences, Inc, Takeda, Trillium Th: Consultancy; Celgene: Research Funding; C4 Therapeutics: Consultancy; Crispr Therapeutics: Research Funding; Daiichi Sankyo: Research Funding; Forty Seven, Inc.: Research Funding; Kura Oncology: Consultancy; Kyowa Hakko Kirin: Consultancy, Research Funding; Millennium/Takeda: Research Funding; Myeloid Therapeutics: Consultancy; ONO Pharmaceuticals: Consultancy; Seattle Genetics: Consultancy, Research Funding; Secura Bio: Consultancy; Shoreline Biosciences, Inc.: Consultancy; Takeda: Consultancy; Trillium Therapeutics: Consultancy, Research Funding; Tubulis: Consultancy; Verastem/Securabio: Research Funding. Izutsu: Daiichi Sankyo: Honoraria, Research Funding; Eisai: Honoraria, Research Funding; HUYA Bioscience International: Research Funding; Kyowa Kirin: Honoraria, Research Funding; Takeda Pharmaceutical: Honoraria, Research Funding; Yakult: Research Funding; AbbVie: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; Bayer: Research Funding; Beigene: Research Funding; Chugai: Honoraria, Research Funding; Genmab: Honoraria, Research Funding; Incyte: Research Funding; Janssen: Honoraria, Research Funding; MSD: Research Funding; Novartis: Honoraria, Research Funding; Ono: Honoraria, Research Funding; Pfizer: Research Funding; Symbio: Honoraria, Research Funding; Allergan Japan: Honoraria; FUJI FILM Toyama Chemical: Honoraria. Ishitsuka: Genzyme: Other: Personal fees; Sumitomo Dainippon Pharma: Other: Personal fees, Research Funding; Eisai: Other: Personal fees, Research Funding; Mochida: Other: Personal fees, Research Funding; Astellas Pharma: Other: Personal fees, Research Funding; Pfizer: Other: Personal fees; Novartis: Other: Personal fees; Janssen Pharmaceuticals: Other: Personal fees; Taiho Pharmaceuticals: Other: Personal fees, Research Funding; Mundipharma: Other: Personal fees; Takeda: Other: Personal fees, Research Funding; BMS: Other; Chugai Pharmaceutical: Honoraria, Other: Personal fees, Research Funding; Celgene: Honoraria, Other: Personal fees; Ono Pharmaceutical: Other: Personal fees, Research Funding; Kyowa Kirin: Other: Personal fees, Research Funding; Daiichi Sankyo: Consultancy, Other: Personal fees; Shire: Other; Otsuka Pharmaceutical: Other: Personal fees; Teijin Pharma: Research Funding; MSD: Research Funding; Asahi kasei: Research Funding; Eli Lilly: Research Funding; Huya Japan: Other: Personal fees. Kato: Daiichi Sankyo: Current Employment; Bristol Myers Squibb: Current equity holder in publicly-traded company. Jin: Daiichi Sankyo: Current Employment, Current equity holder in publicly-traded company. Du: Daiichi Sankyo: Current Employment; Incyte: Other: Spouse's current employer; Nektar Therapeutics: Ended employment in the past 24 months. Inoue: Daiichi Sankyo: Current Employment.